Cadaver "Calamari" Amyloidogenic Fibrin Aggregates
Spike Protein Pathology from Cadavers Exposed to Bioengineered SARS-CoV-2
Kevin W. McCairn, Ph.D., Kevin McKernan Ph.D., Shojiro Kato M.D., Charles Rixey retired (USMC-CBRN)
Abstract
This report presents a preliminary forensic analysis of anomalous fibrin-like aggregates recovered from postmortem human cadaveric samples. Through a combination of gross morphological inspection, cryosection histology, fluorescent staining, scanning electron microscopy (SEM), elemental analysis (EDX), real-time PCR, Raman spectroscopy, and Real-Time Quaking-Induced Conversion (RT-QuIC), the samples were examined for biochemical and ultrastructural features associated with amyloidogenic and protease-resistant fibrin formation. Findings suggest that the clot samples exhibit hallmarks of abnormal protein aggregation consistent with pathological fibrin remodeling, including enhanced autofluorescence, beta-sheet rich domains, dense fibrillar ultrastructure, and spectral anomalies. PCR confirmed the human origin of the tissues, and preliminary evidence of molecular markers associated with recombinant spike protein exposure (SV40 & Ori) was observed. Limitations of provenance, sample control, and chain-of-custody are acknowledged, and further investigation is recommended to establish clinical, pathological, and etiological relevance.
Introduction
Recent reports have drawn attention to the presence of large, rubbery, white fibrin-like clots recovered from postmortem human vasculature. These structures have been described anecdotally by embalmers and morticians and have raised questions regarding their origin, composition, and potential pathological significance. In response, this investigative effort sought to apply rigorous analytical methodologies to determine whether these samples represent a known thrombotic phenotype or reflect novel features associated with emergent pathologies.
It is now broadly recognized that SARS-CoV-2 infection can exert systemic effects beyond its respiratory tropism, with evidence pointing to persistent coagulopathy and fibrin dysregulation. Of particular concern is the potential of the viral spike protein—whether introduced via infection or recombinant delivery platforms such as LNP-mRNA vaccines—to catalyze the formation of amyloid-like fibrin clots. Prior studies have demonstrated that spike exposure can induce fibrin(ogen) conformational changes that resist fibrinolysis and exhibit beta-sheet structures akin to amyloid fibrils.
This report investigates whether the cadaver-derived clots under study bear the morphological and biochemical hallmarks of such aberrant fibrin. Key research questions include: Are the structures of human origin? Do they exhibit features consistent with amyloidogenesis? Can they be differentiated from conventional fibrin emboli or white clots found in known medical conditions?
Through a multidisciplinary forensic approach, this document catalogs the physical and biochemical attributes of the clot specimens, aiming to provide a transparent, methodologically sound foundation for independent review. Given the limitations of the sampling context and absence of complete clinical background, the findings presented here are exploratory and intended to prompt further controlled research.
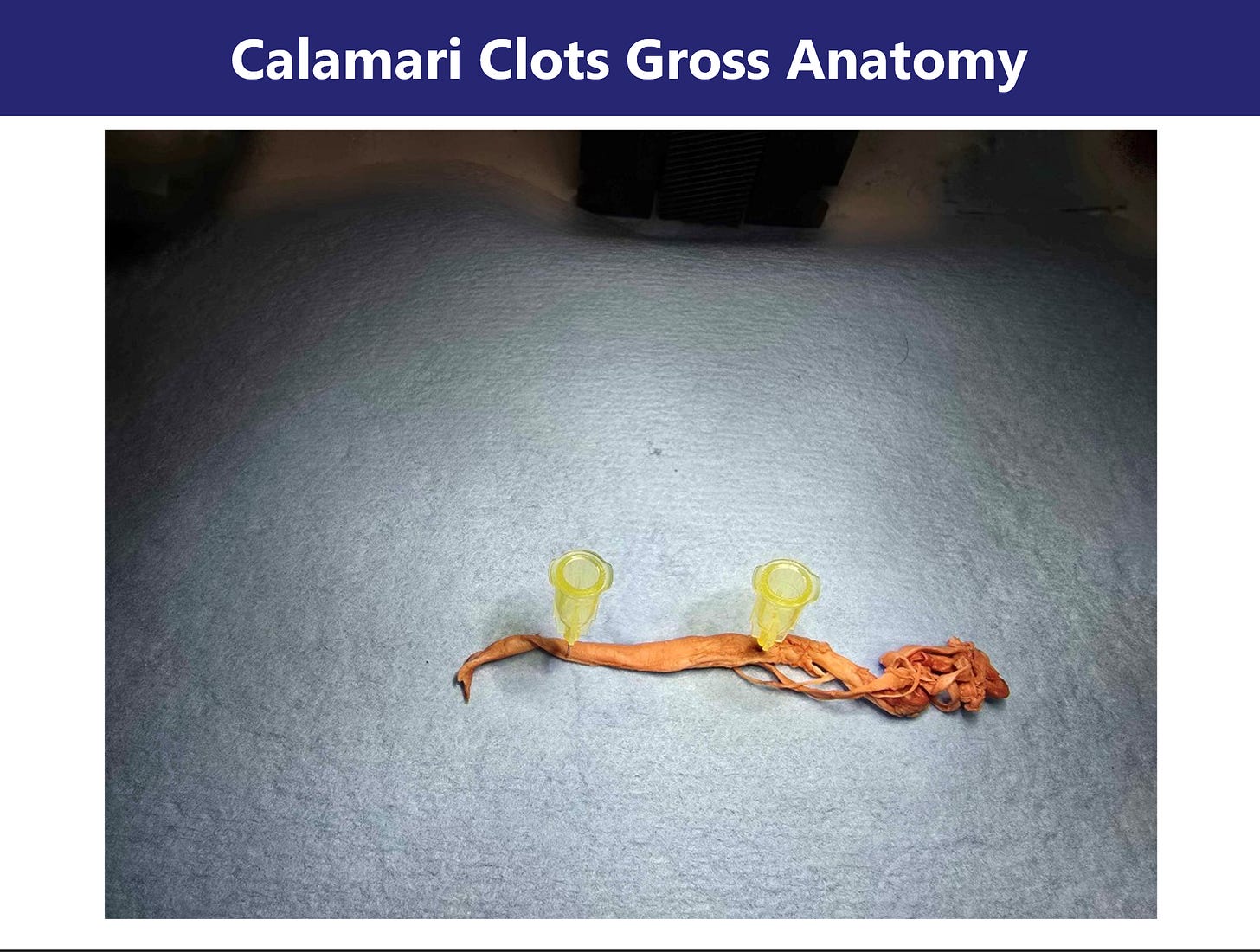
Section 1: Gross Morphology of Clot Structures Image: Slides 1–5
Initial macroscopic inspection of the cadaver-derived clot specimens revealed strikingly anomalous morphology. The samples appear rubbery, fibrous, and frequently coiled or banded—bearing superficial resemblance to marine calamari. Unlike conventional thrombi, these aggregates lack the red-brown coloration and laminar erythrocyte-rich stratification commonly observed in postmortem clots.
Images collected at 4K resolution under dissecting microscope conditions show that the structures retain a solid, tensile integrity. The specimens resist tearing, do not collapse under moderate compression, and maintain structural coherence when manipulated, suggesting a composition distinct from typical fibrin gels. Internal cross-sections show a dense, layered architecture without clear vascular lumens, further differentiating them from vessel-associated thrombi.
These morphological traits suggest either advanced cross-linking within a protein matrix or a structurally aberrant form of polymerized fibrin. The preserved integrity and resistance to manipulation raise the possibility of amyloidogenic remodeling—a hypothesis explored in the following sections through microscopic and spectroscopic analysis.
Section 2: Histology and Fluorescent Imaging of Cryosections Image: Slides 6–11
Tissue samples were cryosectioned at 20 µm thickness and subjected to light and fluorescence microscopy at both 4X and 40X magnification. Hematoxylin-free imaging reveals a heterogeneous, fibrous internal structure with numerous cavities and layered fibrillar regions.
Fluorescence analysis prior to staining revealed strong and uniform intrinsic autofluorescence. Under blue-light excitation, green emission was observed; excitation in the green channel induced red fluorescence. This broad-spectrum response is highly atypical and suggests a high degree of intrinsic molecular ordering or aromatic residue stacking. Such behavior is consistent with cross-linked protein networks or amyloid-like interactions involving tyrosine, tryptophan, or phenylalanine residues.
Thioflavin T (ThT) staining was used to assess the presence of beta-sheet rich amyloid domains. Comparative imaging before and after ThT application revealed discrete "polka-dot" fluorescent foci, as well as diffuse interspersed fluorescence. These data indicate that amyloidogenic stacking is localized but not homogeneous, suggesting microclot heterogeneity within the sample.
Section 3: Scanning Electron Microscopy (SEM) and Elemental Composition via EDX Image: Slides 12–17
Cryosections of 5 µm thickness were prepared for SEM. At low magnification (25X), overall morphology was assessed. Higher magnifications (up to 5000X) revealed a dense reticular meshwork of fibrillar aggregates.
These fibrils display nodular topography and branching interconnections, consistent with pathological protein assembly. Notably, a rotational twist and lateral aggregation features were observed, hallmarks of amyloid fibrin architecture. These ultrastructural findings support the hypothesis that the observed material is not simple polymerized fibrin but instead a protease-resistant, misfolded protein aggregate.
Elemental mapping via EDX revealed high abundance of carbon, nitrogen, oxygen, and sulfur—consistent with proteinaceous material. No significant signal was detected for heavy metals (e.g., Fe, Zn, Cu), ruling out mineral-based aggregation or contamination. The absence of inorganic nucleation points supports an endogenous biochemical origin.
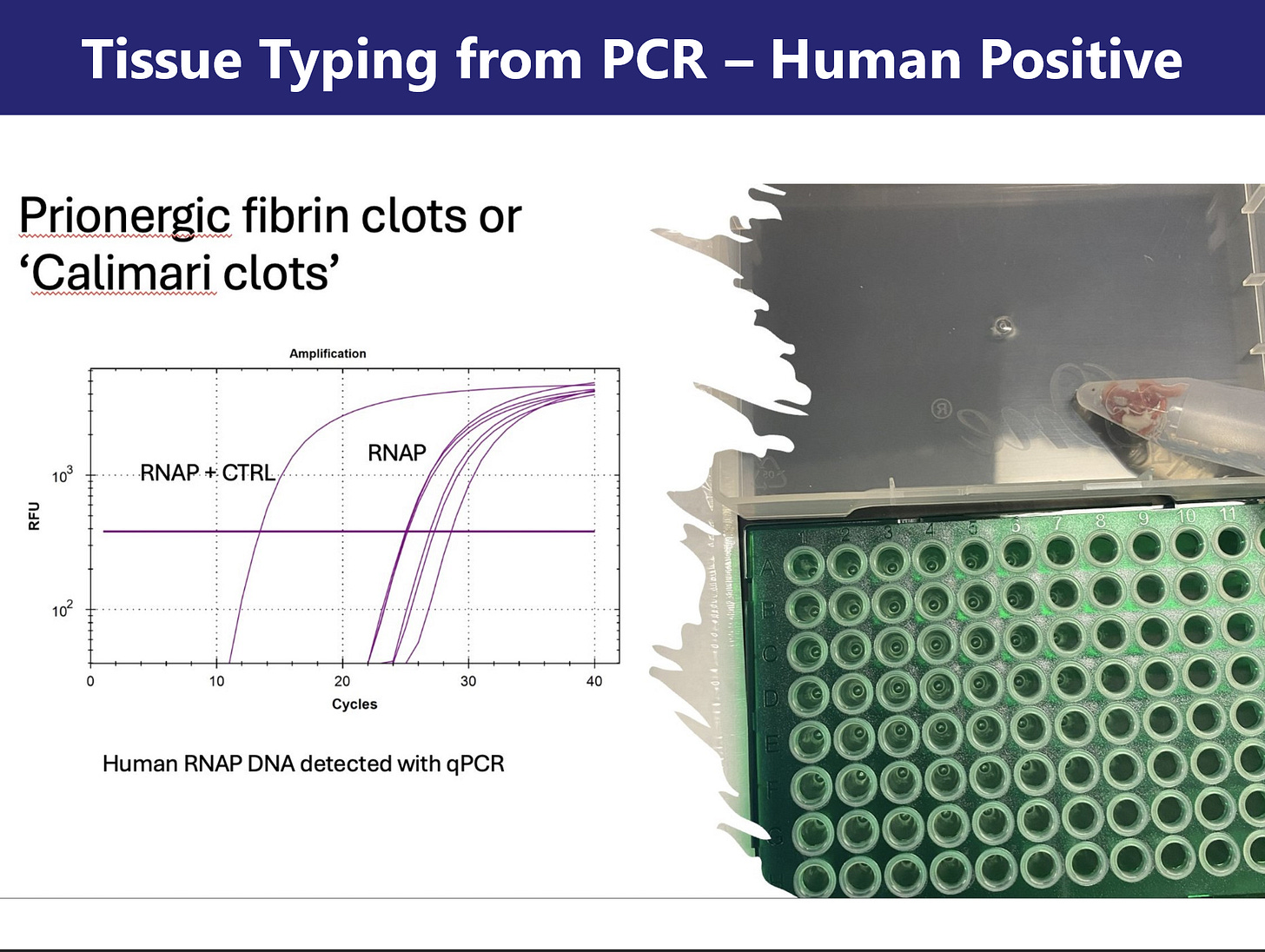
Section 4: PCR and Raman Spectroscopy for Tissue and Molecular Signature Image: Slides 18-20
PCR using primers for the human RNaseP transcript confirmed that the clot-derived samples were of human origin. Additional assays targeting spike protein coding sequences and plasmid-related markers (SV40, Ori) revealed late-cycle amplification, consistent with trace residual presence of recombinant vaccine components. These findings are preliminary and require cautious interpretation.
Raman spectroscopy revealed a major spectral peak at ~1720 cm⁻¹, diverging from the canonical amyloid beta-sheet signature near 1670 cm⁻¹. This spectral upshift may reflect altered secondary structure, ester linkage formation, or protonation of acidic residues. The results point to an atypical fibril composition that may represent a novel polymorph or hybrid aggregate class.
Section 5: RT-QuIC Seeding Activity in Plasma Extracts
RT-QuIC analysis was used to assess seeding potential of the clot-associated material. 3D bar plot visualization of fluorescence intensity across a 96-well plate revealed elevated ThT signal in experimental wells, consistent with fibril formation, when challenged against human plasma.
However, background signal, handling artifacts, and absence of dilution series limit interpretation. Without kinetic rate modeling and control seeding, the data remain suggestive but inconclusive for prion-like activity.
Conclusion
The clot structures described herein exhibit abnormal morphological, histological, ultrastructural, and spectroscopic features. Their dense fibrillar architecture, autofluorescence, ThT reactivity, spectral shifts, and preliminary RT-QuIC activity suggest amyloidogenic remodeling of fibrin under unknown conditions.
While the findings are not definitive, they raise substantial biosafety and pathophysiological questions that warrant immediate, controlled follow-up. The uncertain provenance and unstandardized collection methodology underscore the need for independent replication with verified chain-of-custody.
In the current context of vaccine-induced spike protein exposure and post-infectious complications, these results may point to a novel or under-recognized pathology. As such, they constitute a call for interdisciplinary scientific inquiry, clinical vigilance, and transparent investigation.
Slides





















Dr. McCairn, thank you for your important and tireless work. Great to see an easy-to-share summary here on Substack.
Nice one Doc,
It’s great to see a solid cohort of brilliant minds.
You haven’t missed a beat when it comes to mapping out decent study concepts. I know if you could click your fingers (funds pending) you’d take it to the next level, so here’s to hoping.
For the sake of all those with Long covid and Vaccine injuries I hope the world see’s how important your work is.
Never give up!